20+ Why Is O2 Paramagnetic
Web A magnetic moment is a vector quantity with a magnitude and a direction. Web The MG8G paramagnetic oxygen analyzer measures the concentration of oxygen based on the fact that a magnet attracts gaseous oxygen.

A Temp To Paramagnetic Tempo B Adpa To Adpa O2 Reaction In Presence Download Scientific Diagram
Paramagnetic molecules get attracted towards external magnetic field and diamagnetic repel the external magnetic field.

. An electron has an electron magnetic dipole moment generated by the electrons intrinsic. Web Paramagnetism is due to the presence of unpaired electrons in the material so most atoms with incompletely filled atomic orbitals are paramagnetic although exceptions such as. Solution Magnetic Nature A substances electron configuration can be used to identify its magnetic properties.
Oxygen O2 is paramagnetic indicating 2 unpaired electrons howver simple bonding schemes for O2 with its 12 electrons would. You can easily predict the magnetic nature. Web The reason that it is paramagnetic is because the oxygen molecule has two unpaired electrons.
Web Elemental oxygen O2 is paramagnetic due to unpaired electrons in its molecular orbitals. Web We can explain the paramagnetic nature of oxygen molecule by molecular orbital theory. That is that it is attracted by the magnetic field but does not remain magnetic once it leaves the field.
Web The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. However they incorrectly predicted it to be diamagnetic. Elemental nitrogen N2 is diamagnetic because all the electrons i.
The substance is paramagnetic if its. Actually it is not magnetic but paramagnetic. When you draw molecular orbital diagram of O 2 we can see there are two unpaired.
Web Why oxygen is paramagnetic. Web Class 12 chemistry Ch- 7The p-block elementsTopic- Why is dioxygen paramagnetic12th Chemistry Ch-7 - The p-block Elements. Web Due to the presence of two unpaired electrons we can say that the oxygen molecule is paramagnetic in nature.
To obtain the molecular orbital energy-level. Web One Line Answer Why is O 2 molecule paramagnetic. Electrons not only go around the atom in their orbitals they also spin which.
Advertisement Remove all ads Solution The electronic configuration of O 2 molecule is σ1s 2 σ1s 2 σ2s 2 σ2s 2. The reason why oxygen is paramagnetic is. Web Oxygen Molecule Paramagnetic VSEPR and VBT predicted that O2 is linear and has a double bond.
Web Why is O 2 paramagnetic. Whereas zirconia oxygen analyzers. MOT was used to.

Roshan Yoganathan Senior Project Manager Nutcracker Therapeutics Linkedin
Is O2 2 Diamagnetic Quora
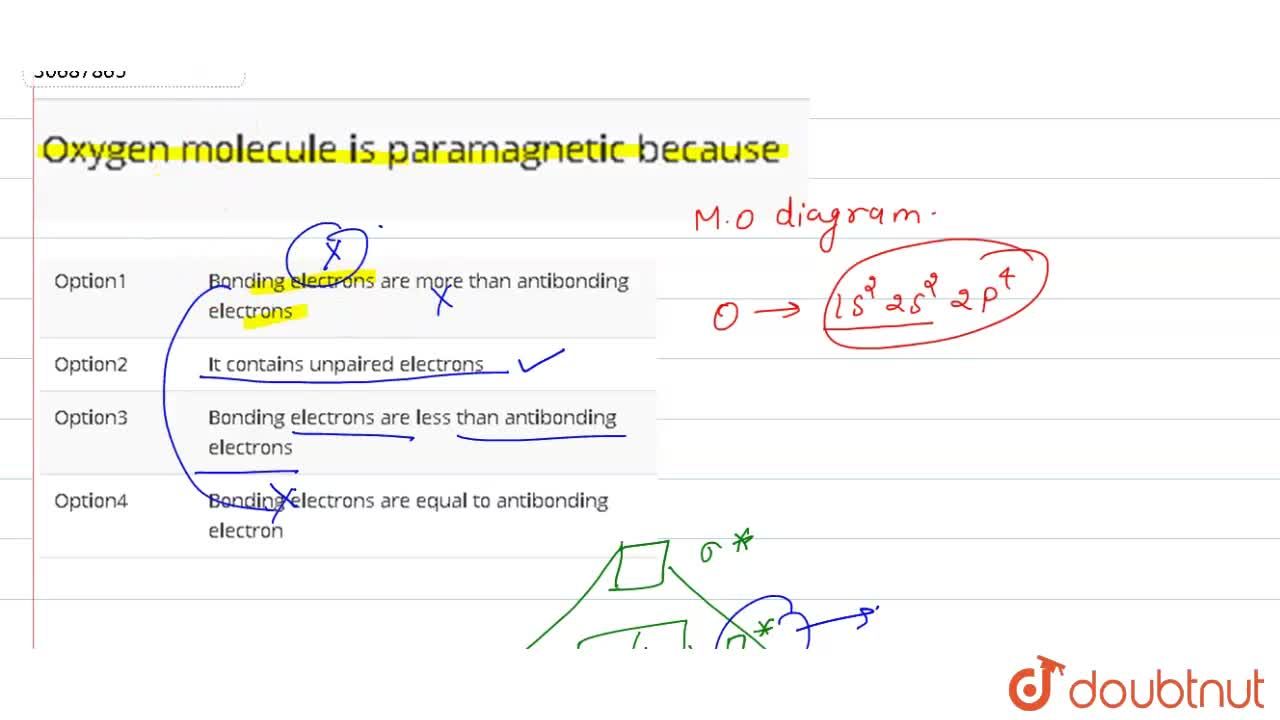
Oxygen Molecule Is Paramagnetic Because

Types Of Analyzers Nox So2 O2 Co Co2 Analyzers By Mangan
Why Is O2 Paramagnetic Socratic
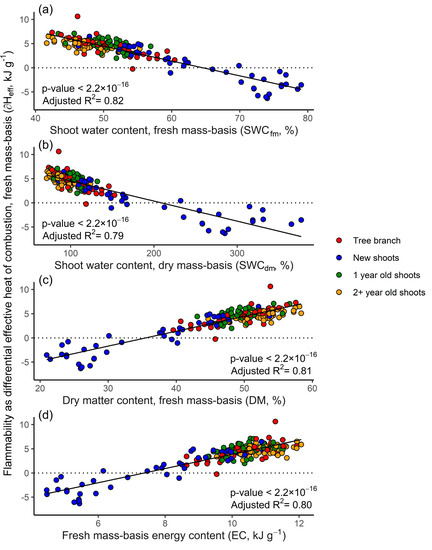
Fire Free Full Text New In Flame Flammability Testing Method Applied To Monitor Seasonal Changes In Live Fuel Html
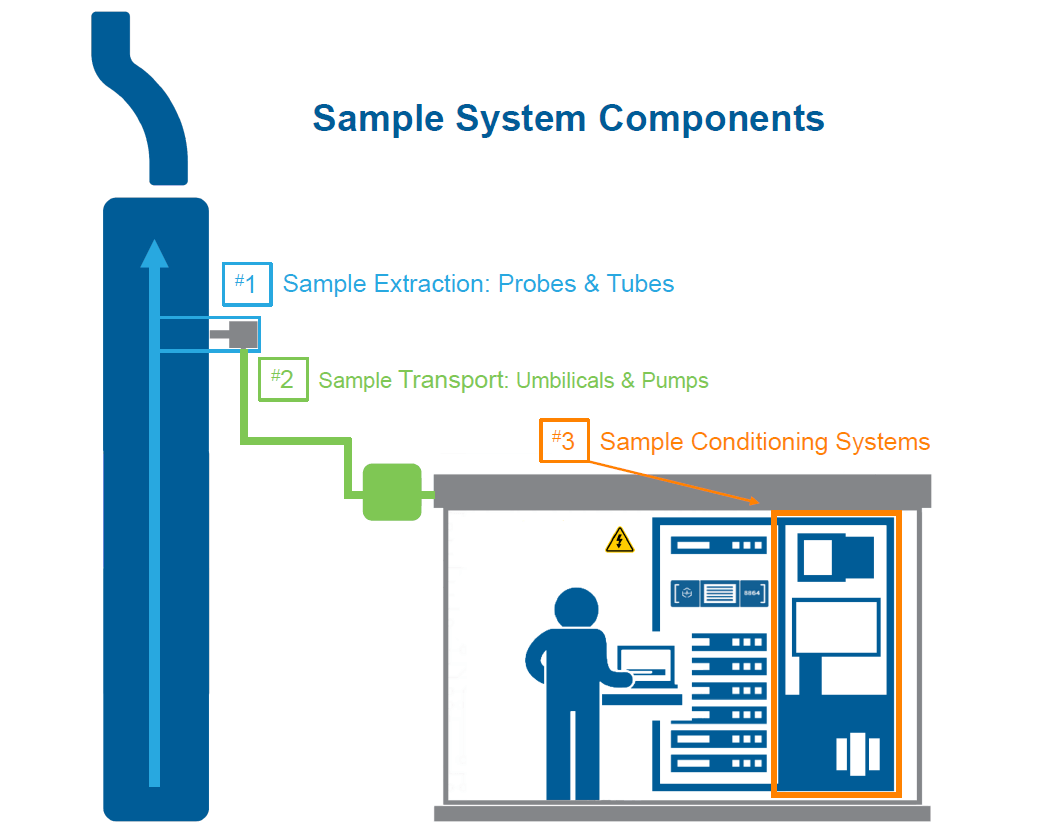
Continuous Emission Monitoring System Upgrade Or Replace

Heteroanionic Materials Based On Copper Clusters Bisphosphonates And Polyoxometalates Magnetic Properties And Comparative Electrocatalytic Nox Reduction Studies Inorganic Chemistry

Analysis Of Spin Frustration In An Feiii7 Cluster Using A Combination Of Computational Experimental And Magnetostructural Correlation Methods Sciencedirect
Color Online The Curie Weiss Linear Fit Of The Inverse Paramagnetic Download Scientific Diagram
Is A Ground State Oxygen A Diamagnetic Substance Or A Paramagnetic Substance Quora

Heterometallic Feiii Ceiv Complexes From The Use Of Aliphatic Aminoalcohol Ligands Sciencedirect
Influence Of Lanthanides On Spin Relaxation And Spin Structure In A Family Of Fe7ln4 Single Molecule Magnets Journal Of Materials Chemistry C Rsc Publishing
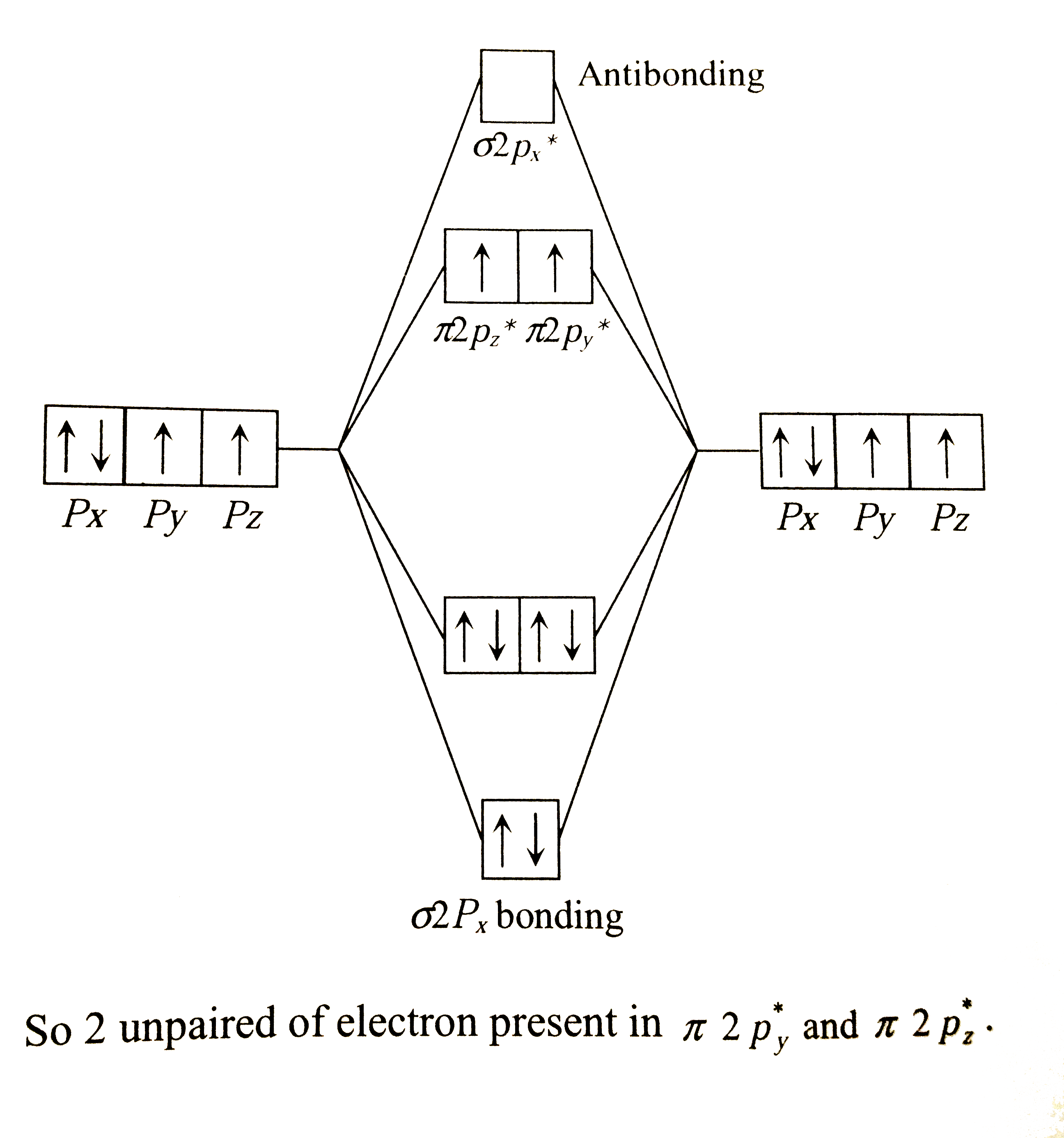
Sigma2p X 1 And Sigma 2p X 1

Day 06 4 Mo Theory O2 Vs N2 Why O2 Is Paramagnetic Youtube

Day 06 4 Mo Theory O2 Vs N2 Why O2 Is Paramagnetic Youtube
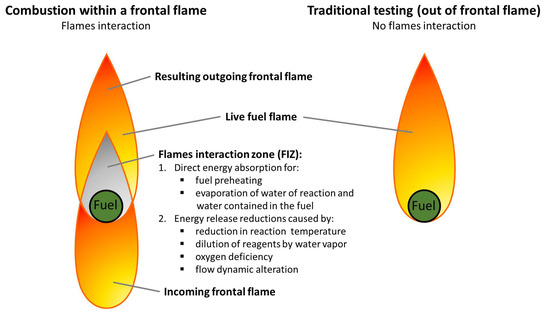
Fire Free Full Text New In Flame Flammability Testing Method Applied To Monitor Seasonal Changes In Live Fuel Html